Redox Titration Between Kmno4 And Feso4 . in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. three types of indicators are used to signal a redox titration’s end point. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. The oxidized and reduced forms of some titrants, such as.
from askfilo.com
in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. The oxidized and reduced forms of some titrants, such as. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. three types of indicators are used to signal a redox titration’s end point. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works.
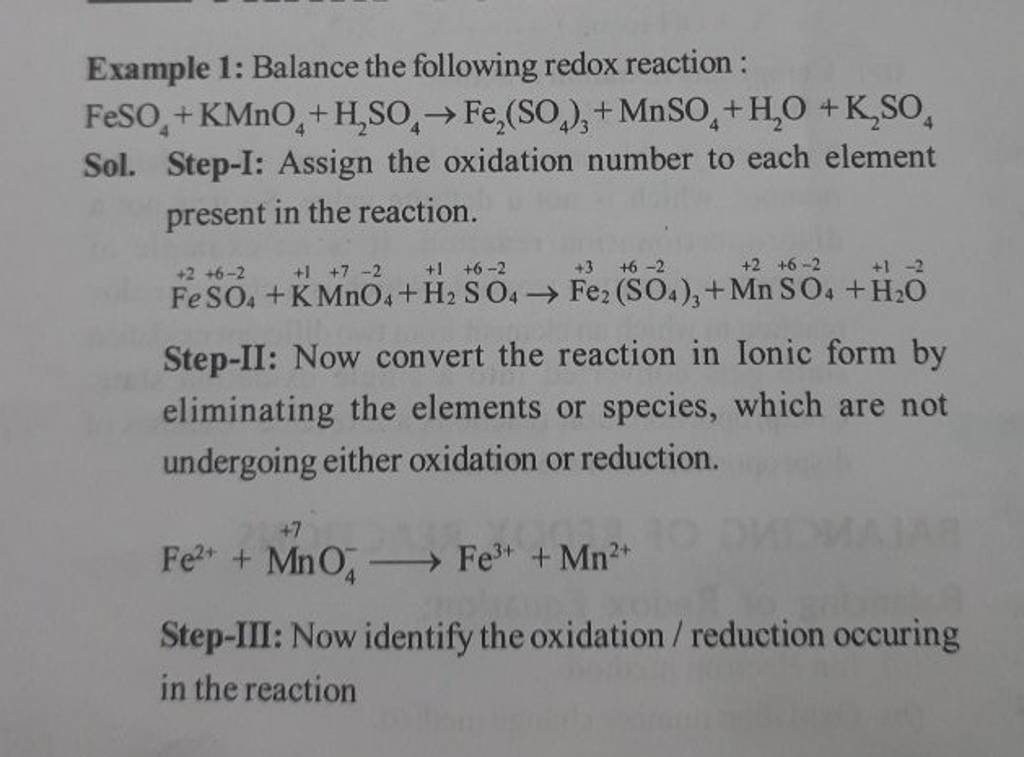
Example 1 Balance the following redox reaction FeSO4 +KMnO4 +H2 SO4 →F..
Redox Titration Between Kmno4 And Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. three types of indicators are used to signal a redox titration’s end point. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The oxidized and reduced forms of some titrants, such as. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance.
From studylib.net
Lab4.3 Redox Titration.doc Redox Titration Between Kmno4 And Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. The oxidized and reduced forms of some titrants, such as. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. three types of indicators are used. Redox Titration Between Kmno4 And Feso4.
From poe.com
What are the products and changes in oxidation states when KMnO4 reacts Redox Titration Between Kmno4 And Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. three types of indicators are used to signal a redox titration’s end point. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. The oxidized and reduced forms of some titrants, such. Redox Titration Between Kmno4 And Feso4.
From www.studypool.com
SOLUTION Redox titration Studypool Redox Titration Between Kmno4 And Feso4 about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. The oxidized and reduced forms of some titrants, such as. . Redox Titration Between Kmno4 And Feso4.
From www.toppr.com
PART VI REDOX TITRATIONS (21) A solution of 10 ml M/10 FeSO4 was Redox Titration Between Kmno4 And Feso4 three types of indicators are used to signal a redox titration’s end point. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. from your description i'd say you were titrating. Redox Titration Between Kmno4 And Feso4.
From studylib.net
Analytical Chemistry lecture note Application of Redox titration Redox Titration Between Kmno4 And Feso4 in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the. Redox Titration Between Kmno4 And Feso4.
From askfilo.com
Example 1 Balance the following redox reaction FeSO4 +KMnO4 +H2 SO4 →F.. Redox Titration Between Kmno4 And Feso4 about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The oxidized and reduced forms of some titrants, such as. from your description i'd say. Redox Titration Between Kmno4 And Feso4.
From xaydungso.vn
FeSO4 + KMnO4 + H2SO4 Hiện Tượng Tìm Hiểu Phản Ứng Hóa Học Kỳ Diệu Redox Titration Between Kmno4 And Feso4 The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox titration’s end point. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of. Redox Titration Between Kmno4 And Feso4.
From www.youtube.com
Redox titration potassium permanganate ferrous sulphate KMnO4 and Redox Titration Between Kmno4 And Feso4 The oxidized and reduced forms of some titrants, such as. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. in the redox titration of $\ce{feso4}$. Redox Titration Between Kmno4 And Feso4.
From poe.com
What is the process of redox titration between KMnO4 and Fe(2)SO4 in Redox Titration Between Kmno4 And Feso4 three types of indicators are used to signal a redox titration’s end point. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. redox. Redox Titration Between Kmno4 And Feso4.
From www.youtube.com
Lesson 28 Redox Reaction of KMnO4 & FeSO4 Topic Redox Reaction Redox Titration Between Kmno4 And Feso4 three types of indicators are used to signal a redox titration’s end point. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The oxidized and. Redox Titration Between Kmno4 And Feso4.
From xaydungso.vn
FeSO4 + KMnO4 + H2SO4 Phản ứng Hóa học và Ứng dụng Thực tiễn Redox Titration Between Kmno4 And Feso4 in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox titration’s end point. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with.. Redox Titration Between Kmno4 And Feso4.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Redox Titration Between Kmno4 And Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. in this experiment you will use a standard solution of. Redox Titration Between Kmno4 And Feso4.
From www.numerade.com
SOLVED Text LESSON Redox Titration Lab Titration Redox Titration Redox Titration Between Kmno4 And Feso4 The oxidized and reduced forms of some titrants, such as. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. three types of indicators are used to signal a redox titration’s end point. about press copyright contact us creators advertise developers. Redox Titration Between Kmno4 And Feso4.
From www.numerade.com
SOLVED Please help to do redox net ionic equation for this reaction Redox Titration Between Kmno4 And Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. three types of indicators are used to signal a redox. Redox Titration Between Kmno4 And Feso4.
From www.youtube.com
Redox titration potassium permanganate ferrous sulphate KMnO4 and Redox Titration Between Kmno4 And Feso4 three types of indicators are used to signal a redox titration’s end point. about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. from your description i'd say you were titrating. Redox Titration Between Kmno4 And Feso4.
From www.youtube.com
Algebraic Method for Balancing KMnO4 + FeSO4 + H2SO4 = K2SO4 + MnSO4 Redox Titration Between Kmno4 And Feso4 in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. in the redox titration of $\ce{feso4}$ with $\ce{kmno4}$, the colour change of the solution at the end point is. three types of indicators are used to signal a redox titration’s end. Redox Titration Between Kmno4 And Feso4.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube Redox Titration Between Kmno4 And Feso4 from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. three types of indicators are used to signal a redox titration’s end point. The oxidized and reduced forms of some titrants, such as. in this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron. Redox Titration Between Kmno4 And Feso4.
From www.meritnation.com
a) In the titration of FeSO4 with KMnO4 in the acidic medium, why is Redox Titration Between Kmno4 And Feso4 redox titration of ferrous sulphate (fe+2) with potassium permanganate kmno4 and calculation of its equivalance. from your description i'd say you were titrating ferrous sulphate, $\ce{feso4}$ solution (the analyte), with. The oxidized and reduced forms of some titrants, such as. three types of indicators are used to signal a redox titration’s end point. in this experiment. Redox Titration Between Kmno4 And Feso4.